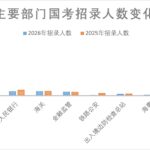
Policy Updates
Three Government Departments Launch Application Process for 2025 High-End Medical Equipment Promotion Projects
A notice has been issued regarding the application process for 2025 high-end medical equipment promotion projects. The initiative aims to implement national health and manufacturing development strategies, accelerate innovation in high-end medical equipment, and facilitate large-scale application of new technologies, products, and scenarios. The program will select projects with leading technological innovation and clinical application levels that demonstrate strong leadership in medical-engineering collaboration, pilot testing, clinical research, iterative upgrades, and promotion. This year’s focus includes promoting 5 key product categories and 4 typical application scenarios.
Drug and Medical Device Approvals
Baicheng Pharma: Innovative Drug BIOS-0623-Z4 Tablets Receive Clinical Trial Approval
Baicheng Pharma announced that its self-developed innovative drug BIOS-0623-Z4 tablets has received clinical trial approval. BIOS-0623-Z4 tablets are a non-opioid targeted mechanism drug for treating cancer pain in adults. Currently, there are no marketed drugs with the same target and indication. BIOS-0623-Z4 tablets are classified as Category 1 chemical innovative drugs that are not marketed domestically or internationally.
Hengrui Pharma: Subsidiary’s HRS-2129 Tablets Receive Clinical Trial Approval for Diabetic Neuropathic Pain
Hengrui Pharma announced that its subsidiaries have received clinical trial approval for HRS-2129 tablets for treating diabetic peripheral neuropathic pain and osteoarthritis pain in adults. HRS-2129 tablets are intended for treating acute and chronic pain. Currently, no domestic drugs with the same target have been approved. The cumulative R&D investment in this project has reached approximately 112 million yuan. Following clinical trial approval, the drug must complete clinical trials and pass regulatory review before it can be marketed.
Bori Pharma: BGM1812 Injection Receives FDA Clinical Trial Approval for Weight Management
Bori Pharma announced that its BGM1812 injection has received FDA approval for Phase I clinical trials for overweight or obesity treatment. BGM1812 injection is a novel long-acting Amylin analog with good molecular activity and pharmaceutical stability. Amylin is a satiety peptide hormone consisting of 37 amino acids, co-released with insulin from pancreatic beta cells. It suppresses appetite by activating brain satiety pathways while delaying gastric emptying and inhibiting glucagon secretion, providing multiple weight loss mechanisms. The weight loss indication for BGM1812 injection has been submitted for clinical trial application in China and is currently under review. Globally, no similar targeted preparations have been approved for weight loss indications.
Capital Markets
Yao Jiasu Completes Tens of Millions in Angel Round Financing
Hangzhou Yao Jiasu Medical Isotope Technology, a hard-tech company dedicated to breaking through GMP-level medical isotope supply bottlenecks, has officially announced the completion of tens of millions of yuan in angel round financing. The funding will primarily be used for core team expansion, technology R&D advancement, and promoting the commercialization of key products including α-nuclide Ac-225.
Industry Events
National Healthcare Security Administration Precisely Investigates Multiple Abnormal Data Cases
In the era of big data, any illegal activities cannot escape “data insight.” Leveraging the national unified healthcare information platform, the National Healthcare Security Administration has precisely investigated multiple typical abnormal data cases, ensuring discovery, investigation, and rectification of each case. The empowering role of healthcare data in supervision continues to become more prominent. The administration released 4 cases of healthcare fund inspections triggered by abnormal data, including: unknown doctors prescribing large quantities of medication, elderly patients undergoing assisted reproduction, batch prescriptions revealing problem clues, and male diseases being treated as female conditions triggering logical contradictions.
Xiangyu Medical Signs Cooperation Agreement with Zhejiang Provincial People’s Hospital to Establish Brain-Computer Interface Ward
The Brain-Computer Interface and Neuromodulation Clinical Research Ward启动仪式暨国际前沿技术研讨会was held. During the event, the Brain-Computer Interface and Neuromodulation Clinical Research Ward was officially unveiled. Xiangyu Medical’s brain-computer interface-neurorehabilitation EMG biofeedback therapy system, brain-computer interface-swallowing nerve and muscle electrical stimulator, and brain-computer interface-ward care system will be deployed in the ward for clinical validation, providing targeted treatment and care support for patients. A cooperation agreement was signed, with future collaboration focusing on clinical research, technology transformation, and talent development in brain-computer interface and neuromodulation.
<p